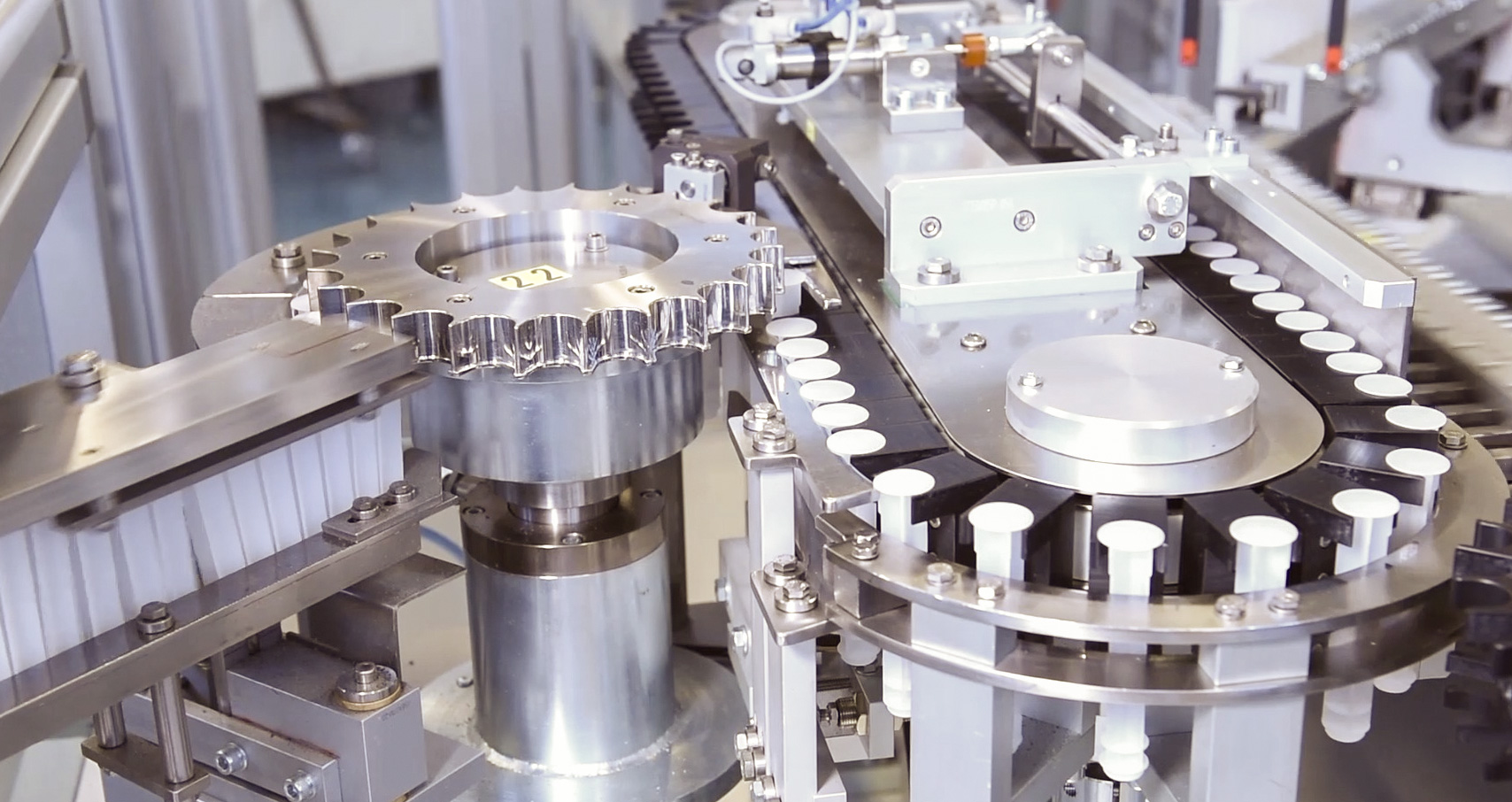
Medical Product Outsourcing (MPO) - 09/2019
Assembling with Reliability and Repeatability Through Automation
An interview with John Wuschner, VP Engineering and Quality, Kahle Automation.
Mark Crawford, Contributing Writer 09.09.19
Assembly and automation are still going strong as medical device manufacturers (MDMs) continue to outsource these services to trusted contract manufacturers (CMs). Needs vary widely from project to project, depending on device design, complexity, materials, production volume, and end-use application. Sometimes the solution is obvious and straightforward; other projects require some creativity and innovation. Automation, in particular, is in high demand for maximizing quality, eliminating variables that impact reliability, reducing costs, and speeding up time to market. Automation manufacturers see the need for advanced equipment and continue to bring out new models with added capabilities that improve assembly operations.
Article excerpt sourced from www.mpo-mag.com, read the full article in September 2019 | Medical Product Outsourcing (MPO) Assembling with Reliability and Repeatability Through Automation
Mark Crawford is a full-time freelance business and marketing/communications writer based in Madison, Wis. His clients range from startups to global manufacturing leaders. He also writes a variety of feature articles for regional and national publications and is the author of five books.