
The Kahle Advantages
Kahle designs and builds custom automation solutions exclusively for the Medical Device and Pharmaceutical Industries. We are dedicated to providing equipment that is designed to operate in a cleanroom environment and can be validated to the latest regulatory standards. Rather than making changes or concessions to meet the medical industry's requirements, we consider those needs as our baseline.
Kahle welcomes the opportunity to partner early on with our customers to provide support during the product design phase. This assures the product and processes for our custom automation solutions are suitable for future automated manufacturing and assists them in their Design for Automation (DFA) process. In addition to providing Proof of Principle (POP) services during the product development stage, we also work closely with our customers to POP high risk areas during the RFQ and preliminary design phases of a project.
Kahle’s approach for the POP, no matter how early or late in the process we have the opportunity to be involved, is to design the tooling and the processes so that they both can be validated at POP with the same process being carried forward all the way through High Speed Automation. This allows our customers to minimize their risk and save precious time and money as the project moves forward.
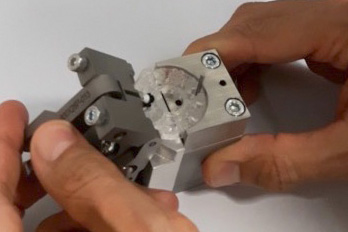
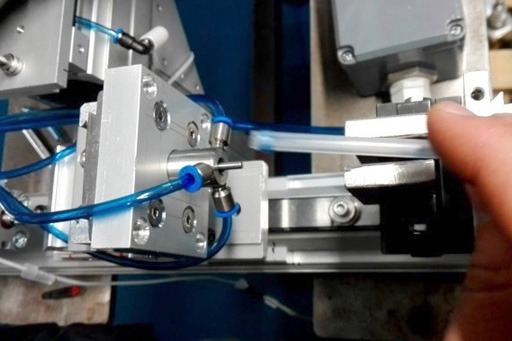
Our design methodology when working on Pilot/Launch Lines is similar to our Proof of Principle (POP) processes where the design of the equipment and the processes are validated and scalable for future high-volume production.
It is our experience that Pilot Lines can be approached in several ways, depending on the complexity of the product and the timing required to launch. The full assembly of the product can be done on the Pilot Line or a customer can choose to automate only the critical assemblies and processes on the equipment and complete the balance of the assembly in a semi-automated method. This is usually determined by the quantity of components that need to be manufactured and the period of time the customer is planning to use the equipment. Keeping in mind there is no correct output speed, we have built Pilot Line equipment with an output of 5ppm and 100ppm. It is dependent on the requirements of the product and the customer.
Kahle Semi Automated Safety Device Assembly Machine and Kahle Launch line for a Micro Needle Assembly


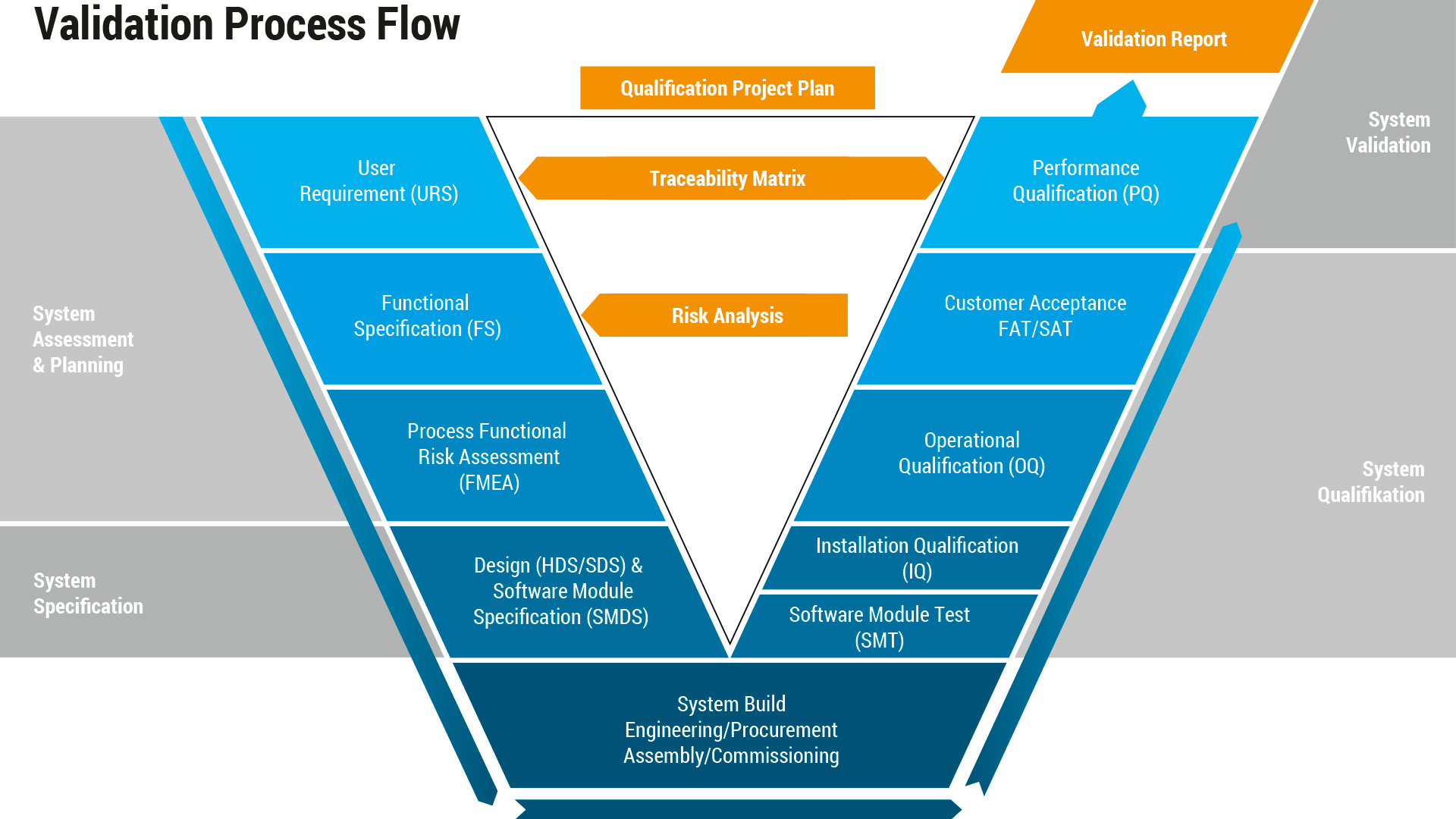
Validation is one of the most important activities for an automation partner. The documentation serves as a framework during equipment design for all mechanical, electrical, and software components of the system and upon machine completion becomes a troubleshooting guide to address any issues that arise during the lifecycle of the equipment.
Utilizing the GAMP5 Guide as a basis, Kahle provides custom validation documentation suited for each customer’s individual requirements, following a “risk-based approach.”
We offer a unique, easy-to-understand approach to validation, designed to be read and understood by program managers, designers, mechanics, and operators alike. This documentation has proven to be an invaluable resource in assessing process and equipment risk during development using input from all customer departments.
To assure the quality of every item that comes off the production line, Kahle performs a comprehensive risk analysis during multiple stages of the project to unearth uncontrolled conditions related to operator safety, machine safety, efficiency, product quality, data integrity, and product user safety. The aim is to reduce risk in the assembly and inspection processes to ensure robust and reliable equipment by using the risk analysis as the underlying foundation of the validation process.
Our validation team is flexible to utilize existing customer internal document templates and structure for ease of integration into existing document control or can deliver a validation package based on Kahle standards. Our Validation Services include development of:
- Quality Plan and / or Validation Plan
- Sequence MAP for all Equipment Processes and Functions
- Functional Requirements Specification
- Design Requirements Specification
- Factory Acceptance Test Protocols and Report
- Installation Qualification Protocols and Report
- Operational Qualification Protocols and Report
- Performance Qualification Protocols and Report
Through our validation process, we enable our customers to achieve and maintain Current Good Manufacturing Practice (cGMP) compliance with all relevant regulatory body requirements.
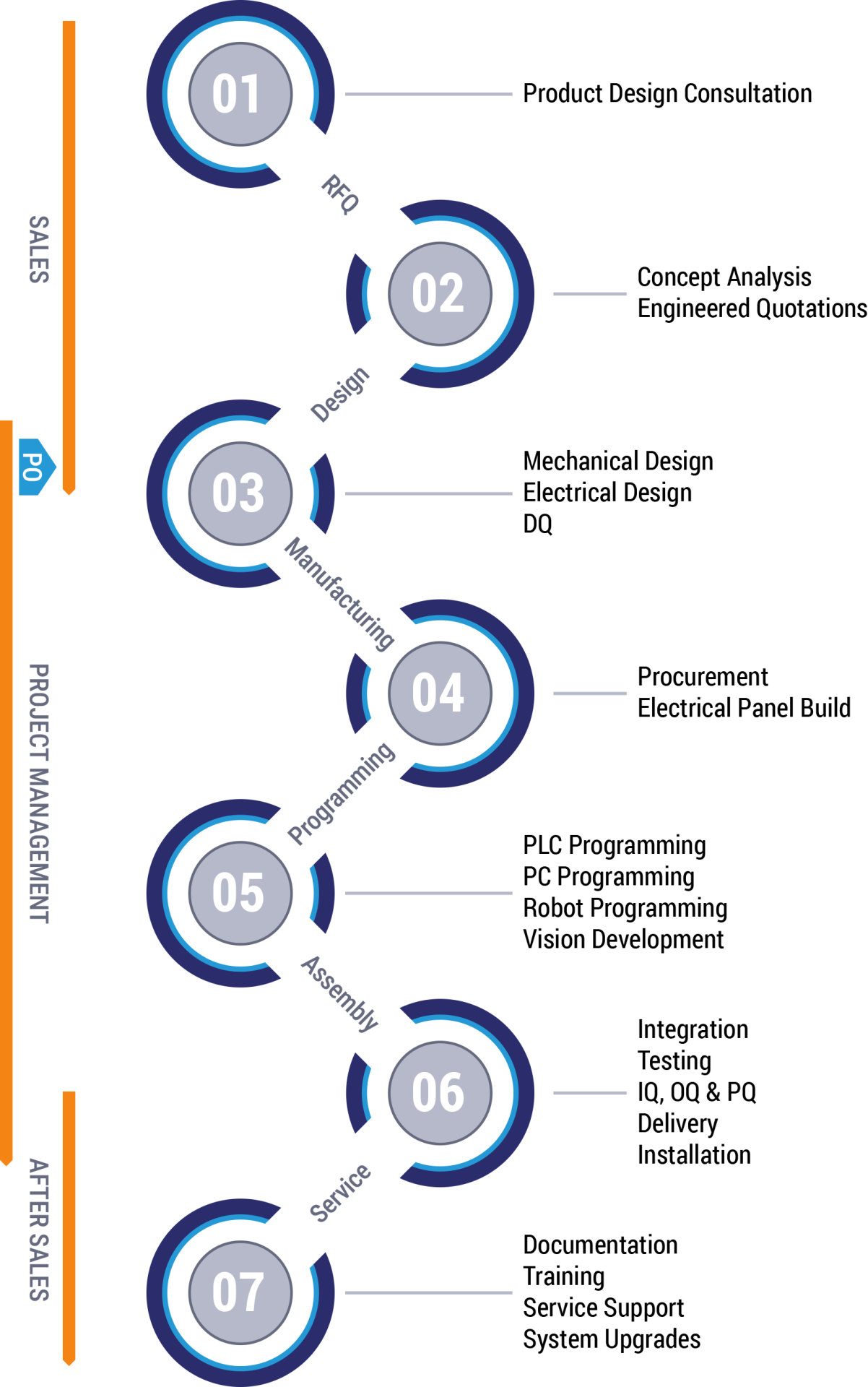
Once an order is placed, all the information gathered in the sales phase is turned over to the engineering department. Design teams, validation members, project managers, and assembly teams are chosen based on their experience with a particular product or process.
The specification for the product is reviewed and the sequence for the assembly and inspection processes are developed along with the traceability matrix based on the agreed to user requirements specification.
Based on the sequence and operation inspected, the FMEA is developed together with the customer’s participation to identify critical requirements as well as risk associated with the project.
Risk areas that have been identified up front or that are as a result of the FMEA review are prototyped to mitigate risk.
The design specification is issued and the machine enters the design phase.
The parts are fabricated, machine is assembled, and debugged to meet the initial output rates and efficiencies.
Our technicians are available for onsite service and installation activities. Kahle utilizes technical support dispatched from our three locations, and for emergency situations can have a technician at your site in 72 hours. Additionally, we provide same day remote access and support for your manufacturing facility anywhere in the world. To expedite the solution and have you back in production, it is Kahle’s policy to provide technical support by the members of the team that were involved in the construction and debug of YOUR equipment. This allows for prompt and efficient solutions with technicians that are familiar with your product and your personnel.
Kahle provides factory training and on-site training for all customer personnel, both technicians and engineering. In addition to formal training sessions we also offer the opportunity for your maintenance team to come and observe the construction and debug of your machine which allows them a deeper understanding of all the aspects of its operation and how to properly maintain and troubleshoot it once it is installed.
All machines are provided with a complete Recommended Spare Parts list and supplier details for all commercial parts so they can be sourced locally should you choose. Our priority is to minimize downtime and not keep our customers waiting for parts.
Kahle also provides Service Contracts to perform Regularly Scheduled Maintenance visits by Kahle Technicians.
Need a custom automation solution?
Kahle is here to help. Contact us today to discuss how we can help you.






