Combination Pharmaceutical Device
Combination products are defined in 21 CFR 3.2(e): A product comprised of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are physically, chemically, or otherwise combined or mixed and produced as a single entity.
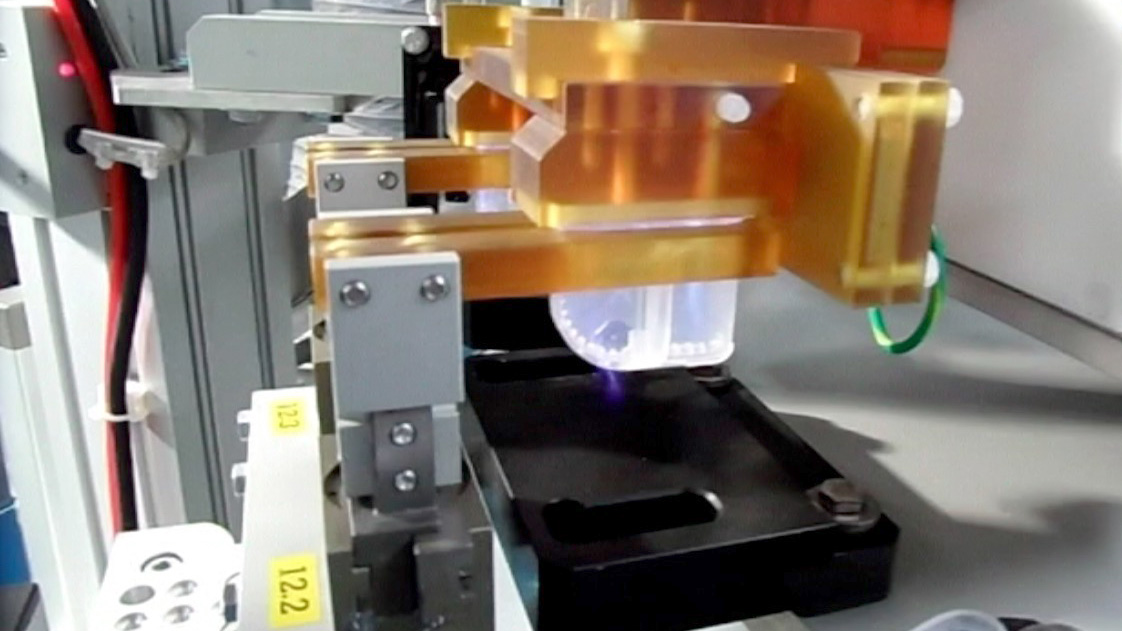
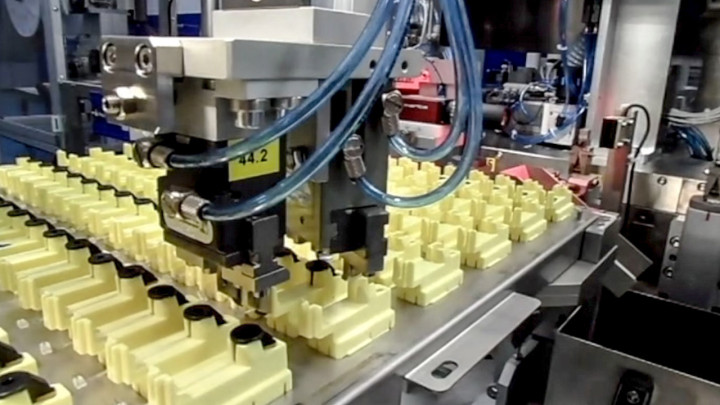
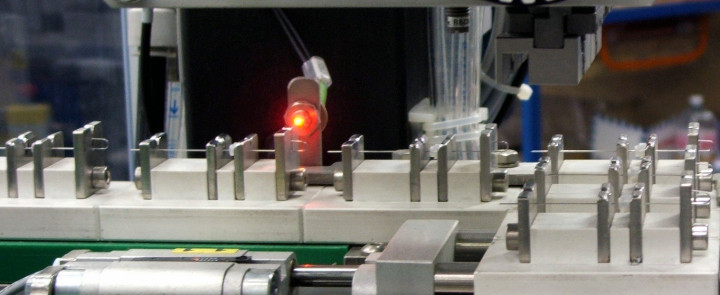
Kahle is a leader in providing automation solutions to the medical device and pharmaceutical industries. As these industries continue to evolove and the methods of drug delivery change so has the reliance on combination pharmaceutical devices and the need to automate manufacturing. Our customers have come to us to assist them as they move forward with the next evolution of drug delivery products.
Kahle has diligently been working on custom automation solutions for this new and challenging market, starting with providing equipment for the manufacture of Wearable Pumps in 2005. Since then, we’ve worked hard to distinguish Kahle as the company that has the technology to provide the solutions for the many different manufacturing requirements of combination pharmaceutical device assembly, such as the intricate needs of the micro fluid paths often required for these systems.
Kahle has also had the opportunity to provide equipment for the manufacture of many the complex Prefilled Glass Safety Syringes. In addition to providing the assembly automation, Kahle has been project lead for the integration of all the other equipment required such as the inspection, washing, filling, depyrogenation, tub filling and packaging, which allows our customers to increase overall line efficiency and output.
For information
on our Custom Medical Device Automation Equipment.